LeChatelier's Principle: Iron(III) Thiocyanate Equilibria . Looks like Tang, tastes like going to the hospital to get your stomach pumped . Chemicals and Equipment Needed • LeChatelier's Principle Kit - O2 o Dropper bottle of 0.10 M Fe(NO 3) 3 • o Dropper bottle of 0.10 M KSCN o Dropper bottle of 0.10 M AgNO 3 o Small vial of NaF (s). In the experiment described below, you will mix dilute solutions of iron(III) nitrate and potassium thiocyanate. Problem 1: Which ions are contained within these solutions? Give their formulae and names. Dissolved iron(III) ions and thiocyanate ions combine to form a so-called complex. A complex consists of a positive ion (= central ion.

Iron (III) nitrate and potassium thiocyanate MVI 1828 YouTube

Solved Experiment 2, Part 1 Iron(III) nitrate and potassium
![SOLVED Concentration of iron(III) nitrate [Fe(NO3)3] (M) 0.20000 Color of iron(III) nitrate SOLVED Concentration of iron(III) nitrate [Fe(NO3)3] (M) 0.20000 Color of iron(III) nitrate](https://cdn.numerade.com/ask_images/14220882a7b444129a38c31a16b7bb26.jpg)
SOLVED Concentration of iron(III) nitrate [Fe(NO3)3] (M) 0.20000 Color of iron(III) nitrate

Photographers' Formulary Potassium Thiocyanate 100 101090

Iron Iii Thiocyanate

In the reaction of potassium thiocyanate and iron (III) chloride, dark red Fe(SCN)3 forms, which

SOLVED The Reaction of Iron (III) Nitrate with Potassium Thiocyanate In this experiment, we
Potassium Thiocyanate VS Iron III Choride YouTube

Potassium Thiocyanate, Purified, Spectrum Chemical, Quantity 500 g Fisher Scientific

Iron Nitrate and Potassium Thiocyanate YouTube

C. Iron(III)Thiocyanate Equilibrium Fe (aq)+ SCN

Solved Experiment One Watch the video describing an

Iron Nitrate Solution
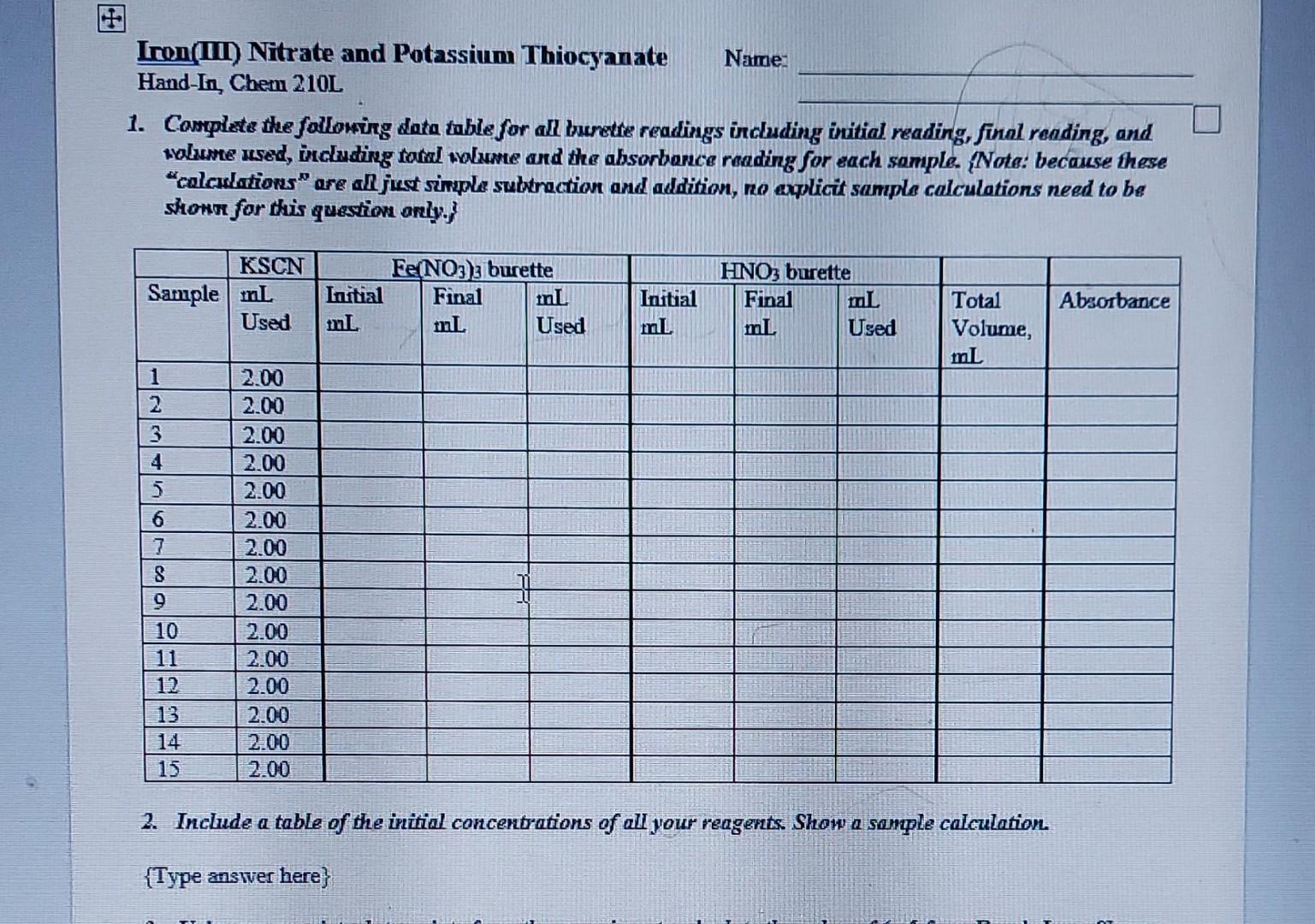
Iron(III) Nitrate and Potassium Thiocyanate HandIn,

Iron III Chloride Reaction With Potassium Thiocyanate (FeCl3 + KSCN) YouTube

Solved In This Experiment, The Equilibrium Between Fe^3+,...
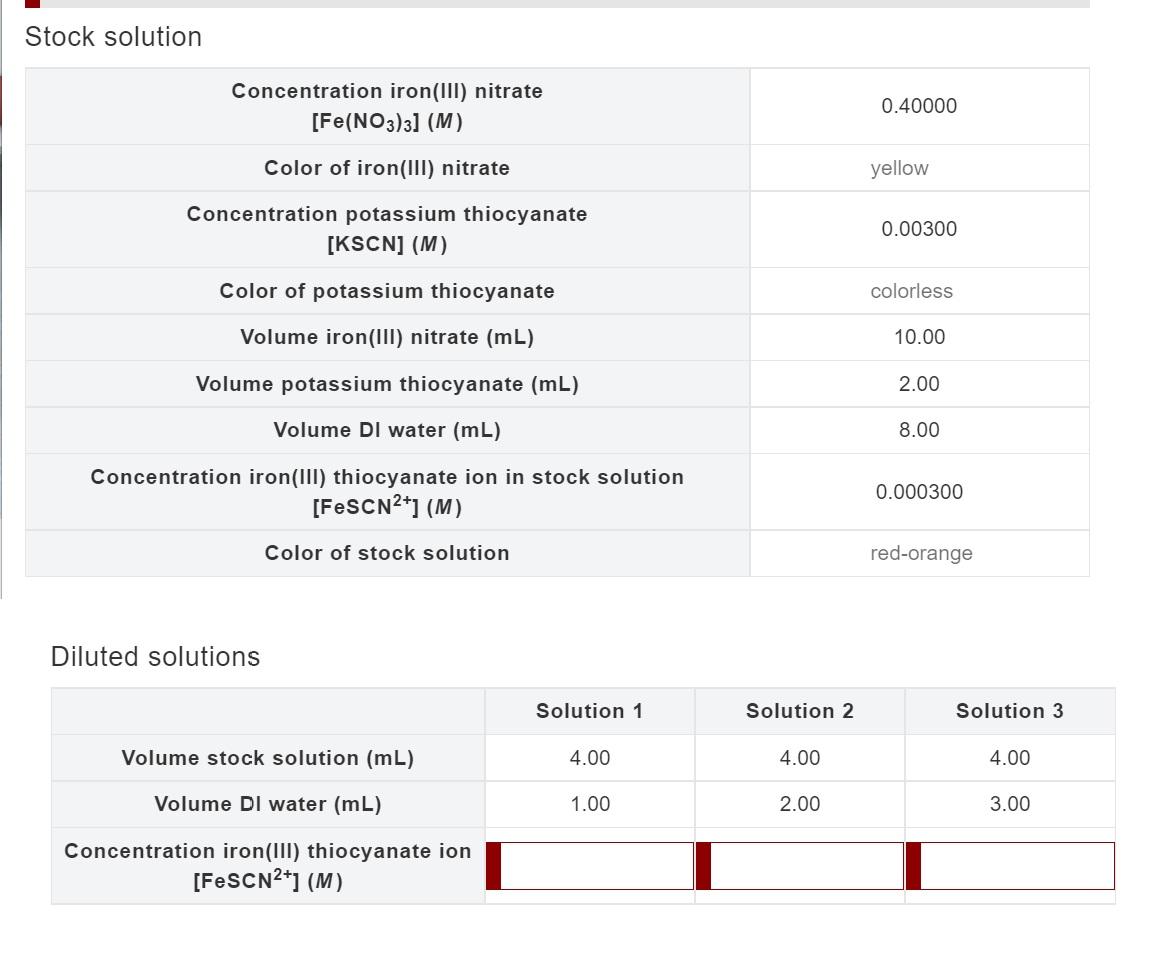
Solved Stock solution Concentration iron(III) nitrate
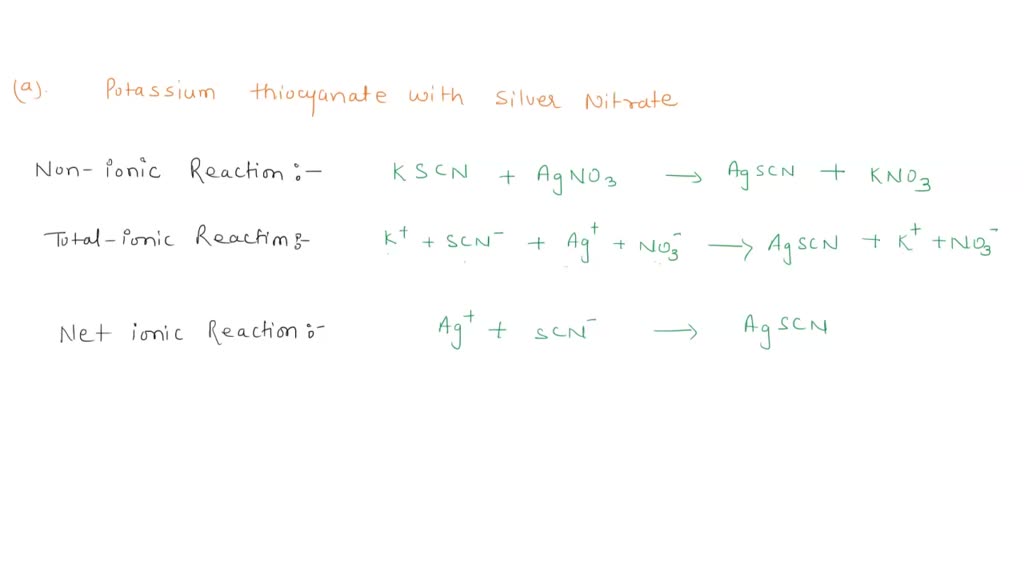
SOLVED Write the nonionic, totalionic, and net ionic equations for a) potassium thiocyanate
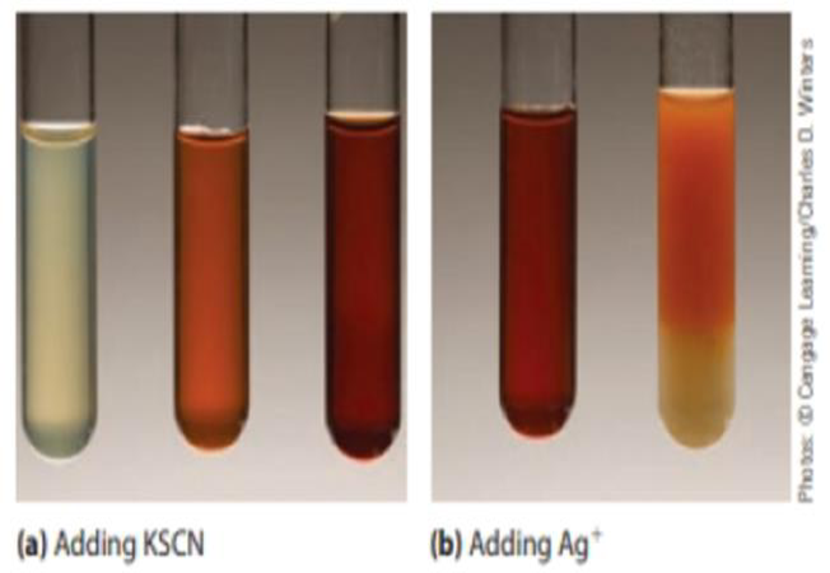
The photographs below (a) show what occurs when a solution of iron(III) nitrate is treated with
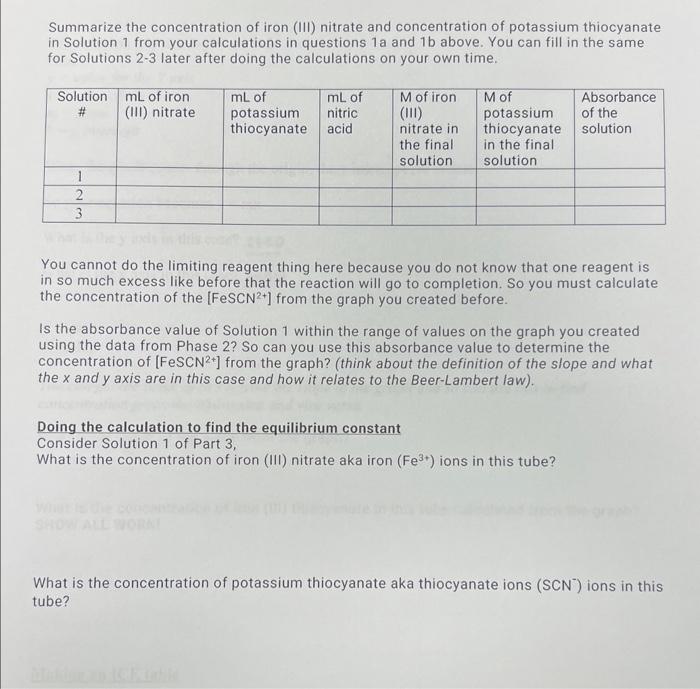
Solved Summarize the concentration of iron (III) nitrate and
To test tube #2, add 1 mL of 0.1 M iron(III) chloride (FeCl 3) solution. Record your observations. To test tube #3, add 1 mL of 0.1 M potassium thiocyanate (KSCN) solution. Record your observations. To test tube #4, add 0.1 M silver nitrate (AgNO 3) solution drop by drop until a distinct color change occurs. Record your observations.. The important players in the reaction are the Fe 3+ from the iron compound and the K + and SCN 2+ from the potassium thiocyanate. These ions form a red-colored iron(III) thiocyanate complex [KSCN=Fe(SCN)₃ ]. It's red like the iron-based hemoglobin complex that gives real blood its color, minus any injury. The color of iron thiocyanate.