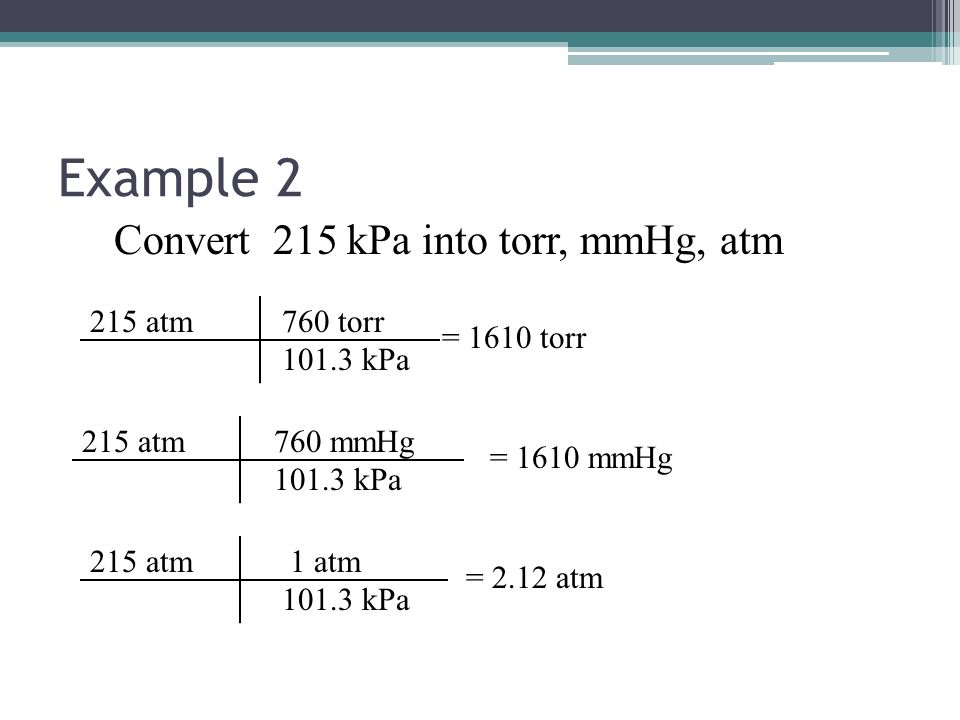
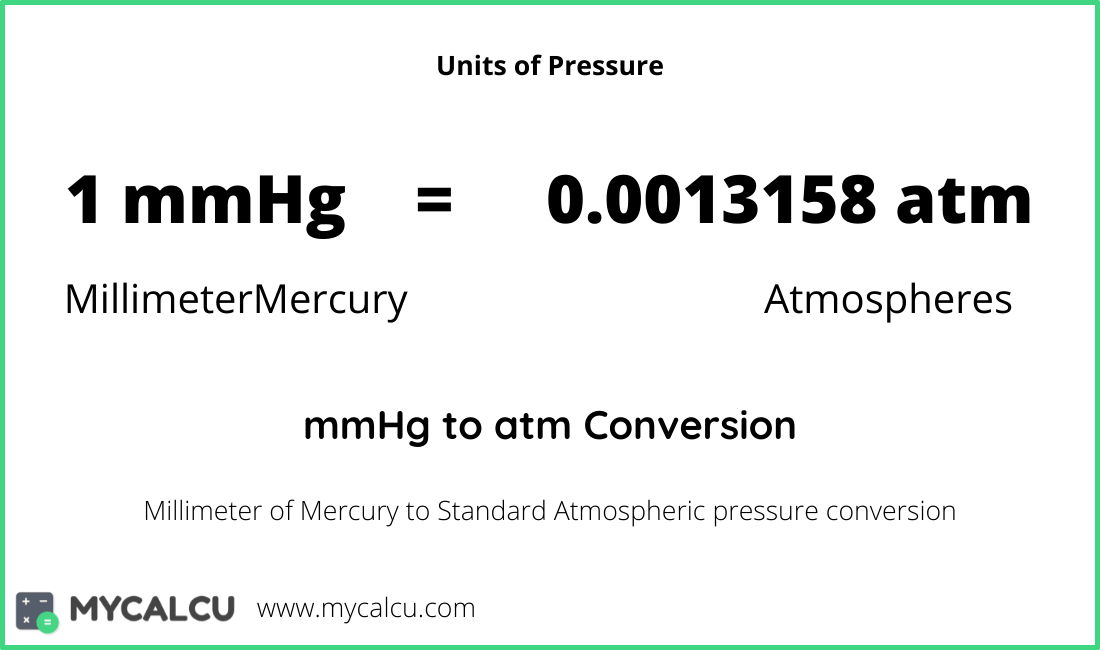
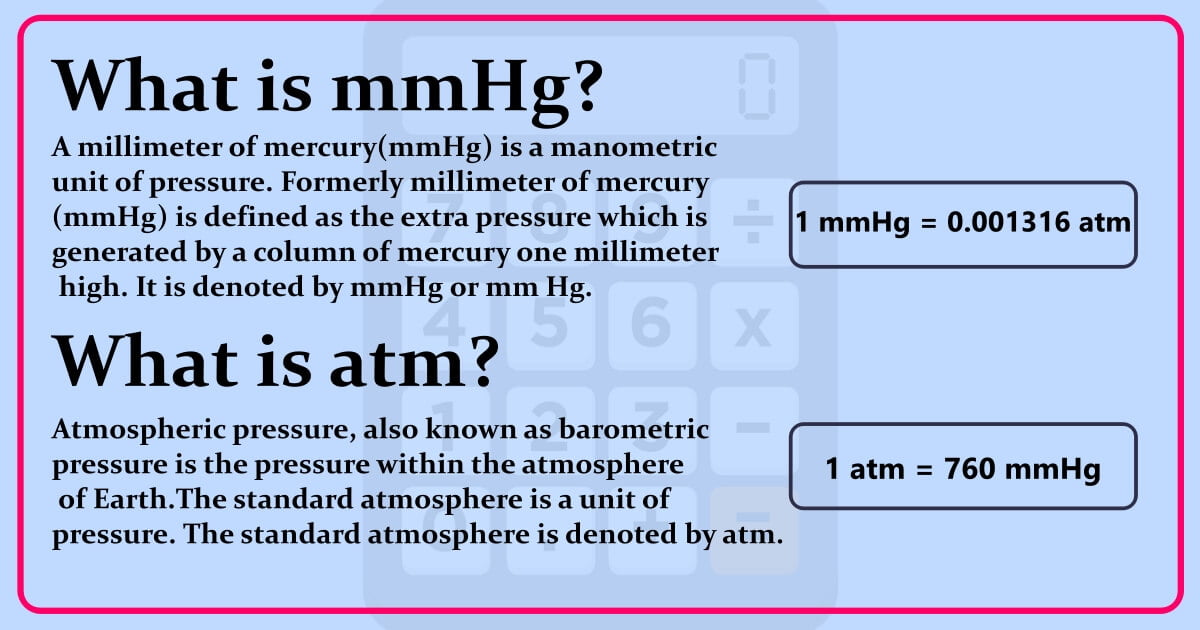
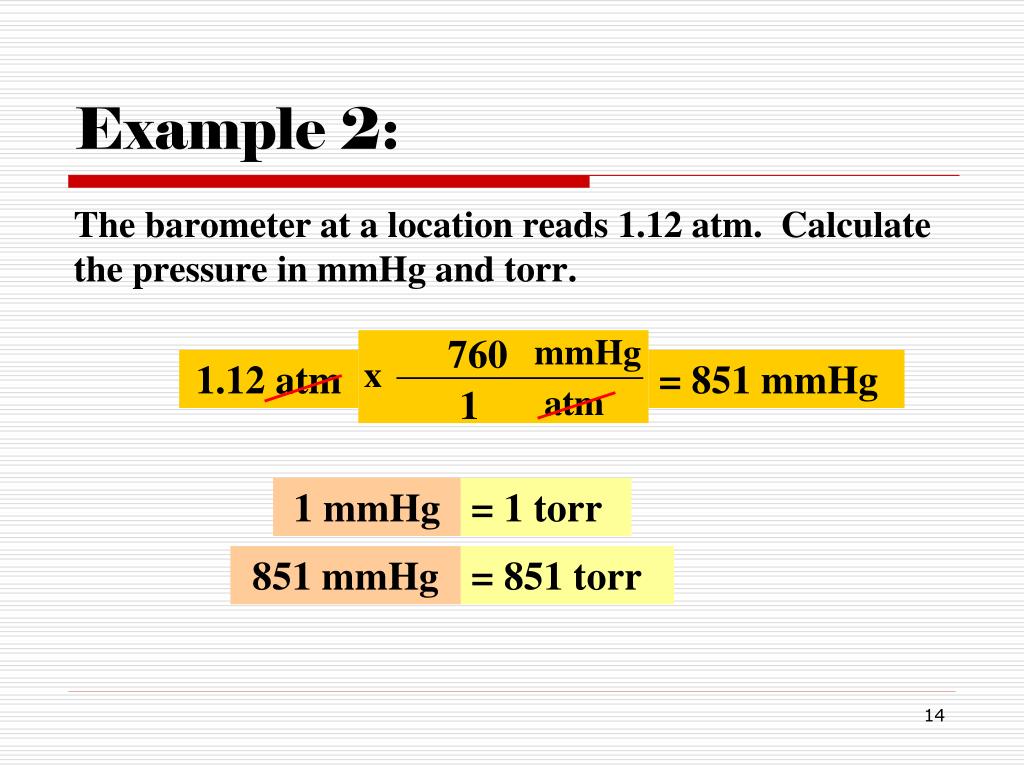
The millimeter of mercury by definition is 133.322387415 Pa (13.5951 g/cm 3 × 9.80665 m/s 2 × 1 mm), which is approximated with known accuracies of density of mercury and standard gravity. The torr is defined as 1 / 760 of one standard atmosphere, while the atmosphere is. Conversion chart millimeter mercury [0 °C] to atmosphere [standard] (mmhg to atm): Millimeters Of Mercury: Atmospheres: 1 mmHg. 0.0013157897853442 atm. 2 mmHg. 0.0026315795706884 atm. 3 mmHg. 0.0039473693560326 atm.
De Mmhg A Atm Estudiar
Mmhg to atm calculator deltaaqua

Mmhg to atm formula managedelta
mmhg to atm Pressure Conversion

1520 mmhg to atm holdengc

How to convert mm of hg into atm? YouTube
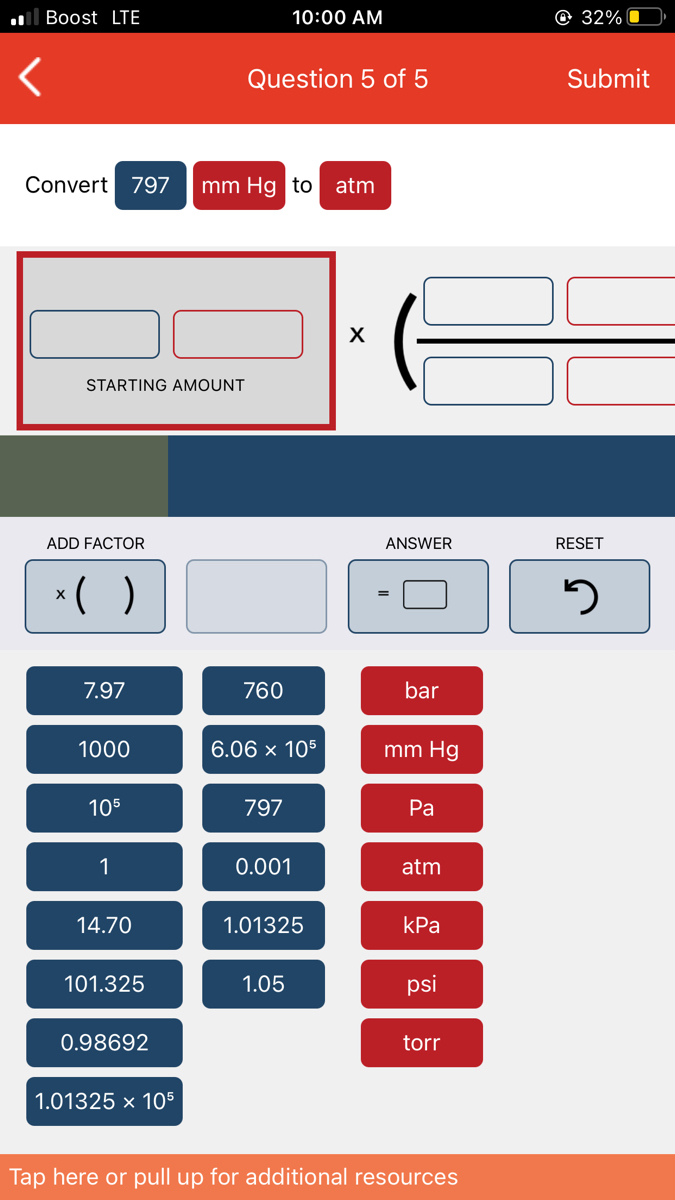
Answered Convert 797 mm Hg to atm STARTING… bartleby

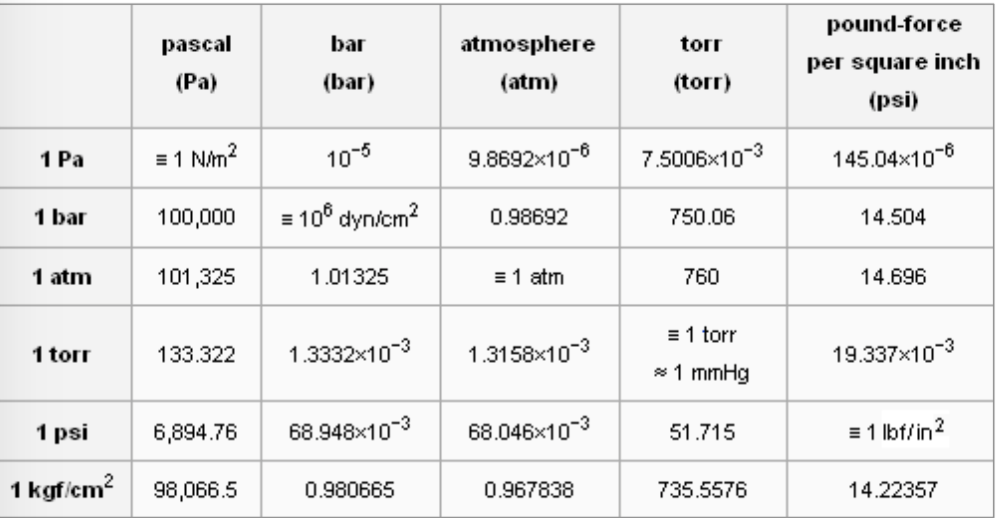
Gas Pressure Unit Conversions torr to atm, psi to atm, atm to mm Hg, kpa to mm Hg, psi to torr

250 mmhg to atm columbuslokasin
conversion of atm to mmhg Easy to convert CalculatorPort
Conversion factor for mmhg to atm lulimidnight
Converting mmhg to atm costlikos
PPT Chapter 11 Gases PowerPoint Presentation, free download ID1586364
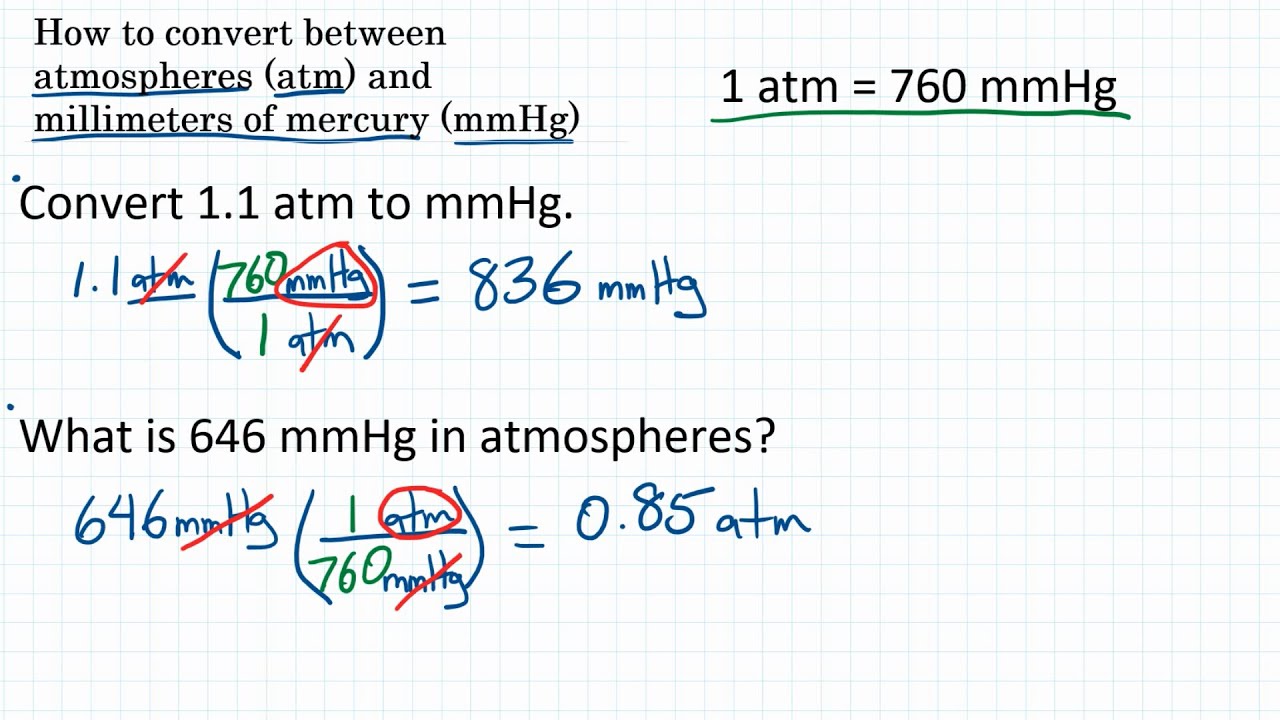
How to Convert Pressure Units atm & mmHg YouTube

SOLVEDMake the indicated pressure conversions. a. 1.54 \times 10^{5} Pa to atmospheres b. 1.21

Mmhg to atm converter bingeropia
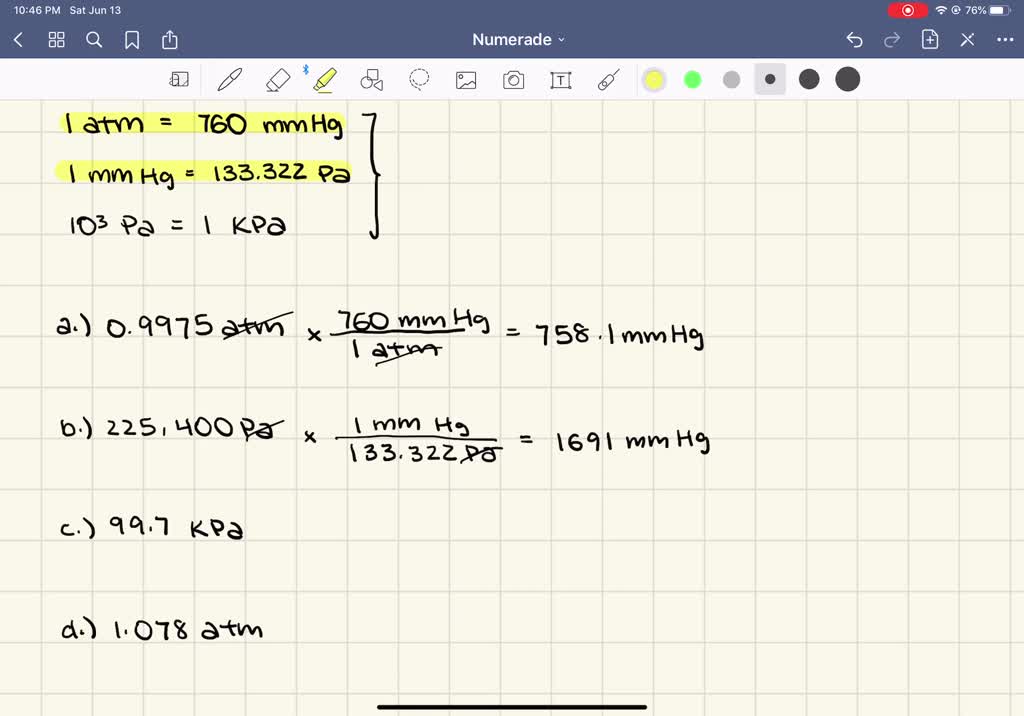
Mmhg to atm formula managedelta
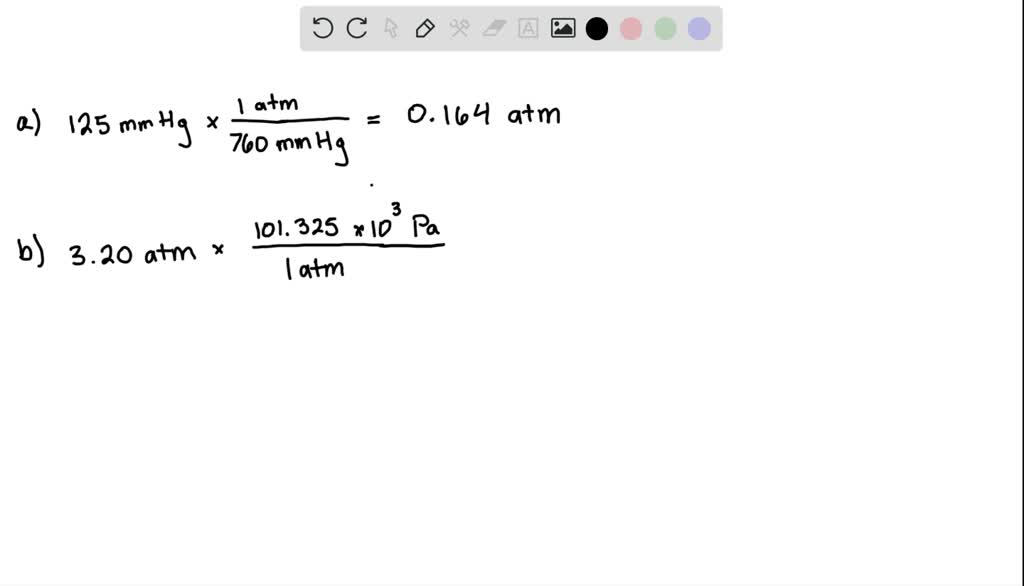
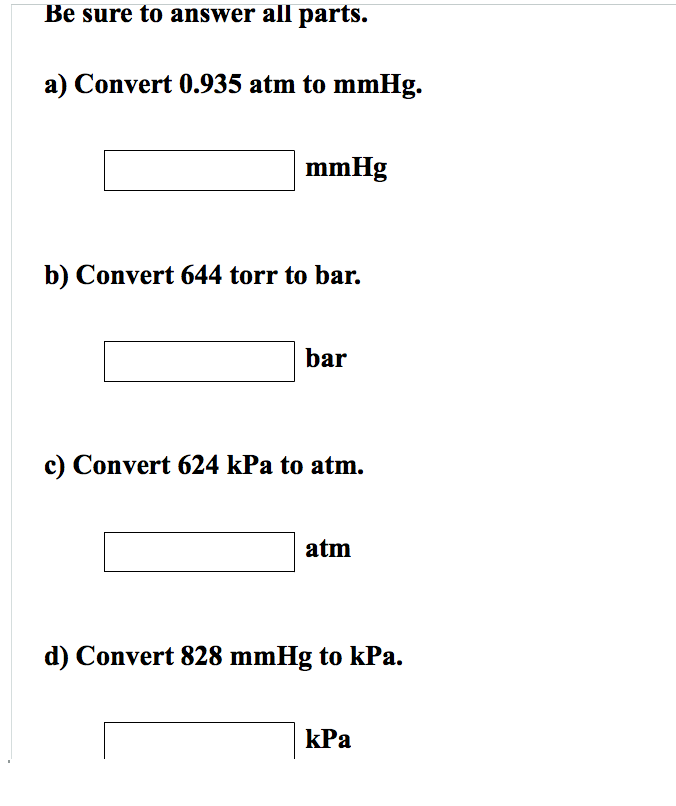
SOLVEDConvert each of the following into the unit specified. a. 125 mm Hg into atmospheres b. 3

Como Pasar De Mm Hg A Atm Estudiar
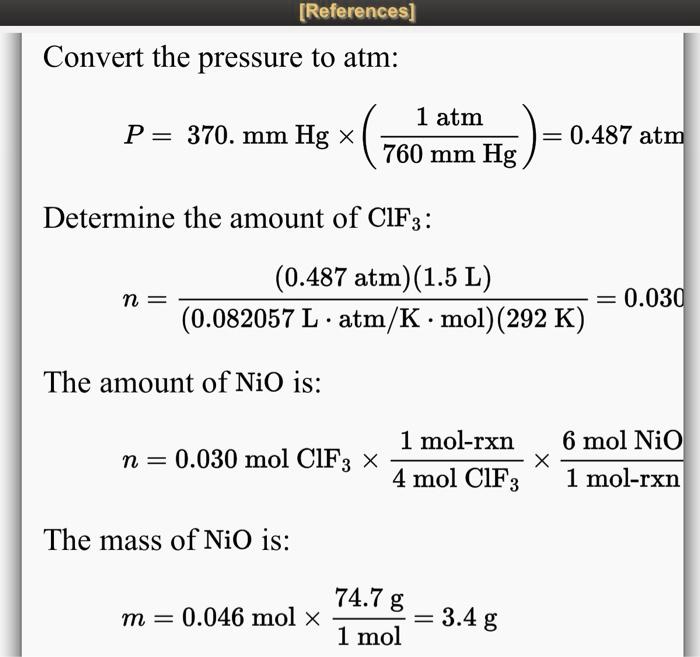
SOLVED Convert the pressure to atm 1 atm P = 370 mm Hg X 760 mm Hg / 760 mm Hg = 0.487 atm
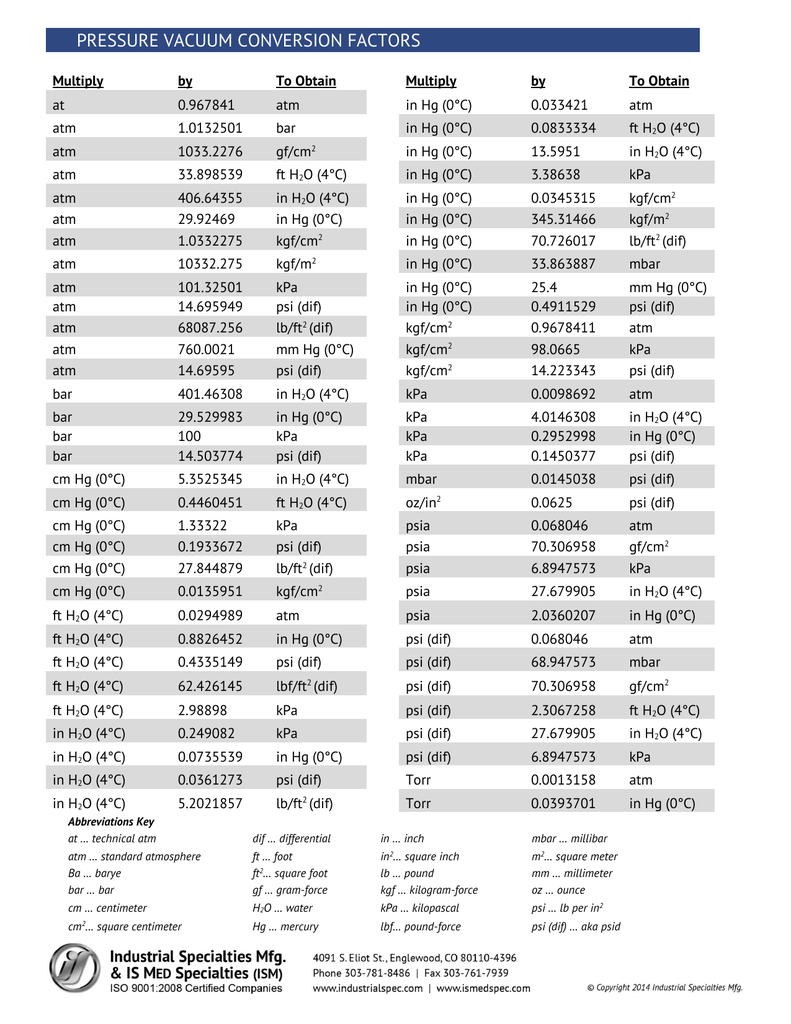
This tool converts one pressure unit to other pressure unit. The available pressure units are atmosphere (atm), bar, millibar (mbar), pascal (pa), kilopascal (kpa), pound per square inch (psi), pound per square foot (psf), torr, millimeter of mercury (mmhg). 1 atmospheres = 760 millimeter of mercury Formula atmospheres in millimeter of mercury.. Understanding the basics of mm to ATM converter: Millimeters of mercury (mmHg): This unit of pressure measurement is commonly used in meteorology and medicine, particularly in measuring blood pressure. It is based on the amount of pressure exerted by a column of mercury one millimeter high.