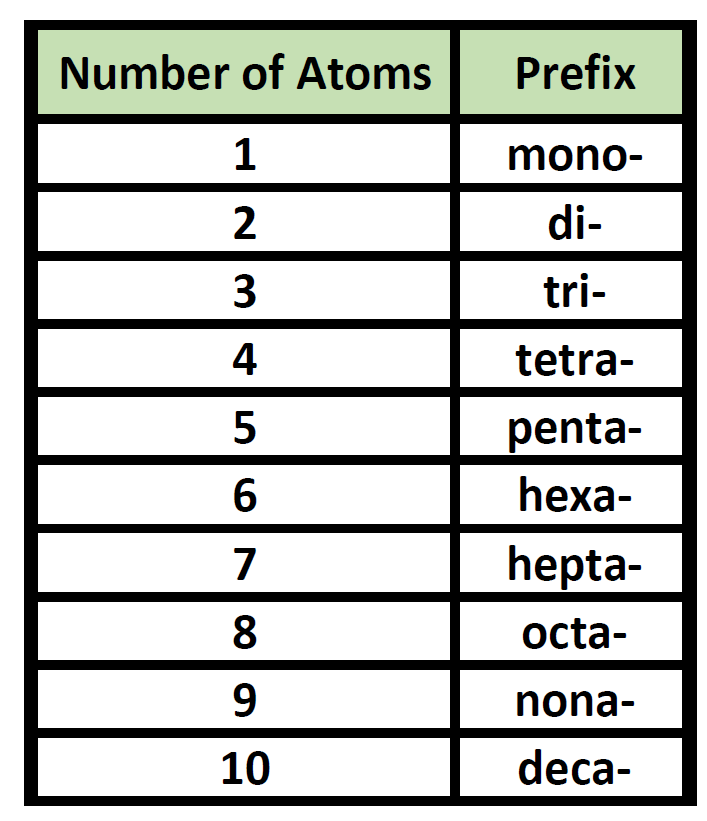
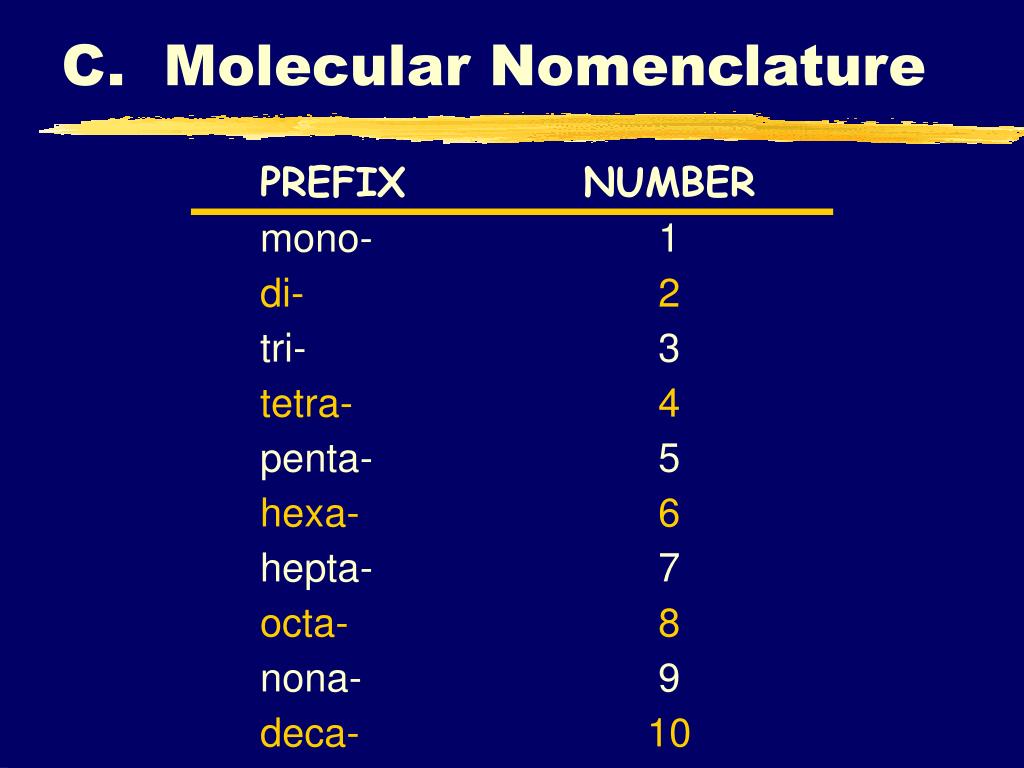
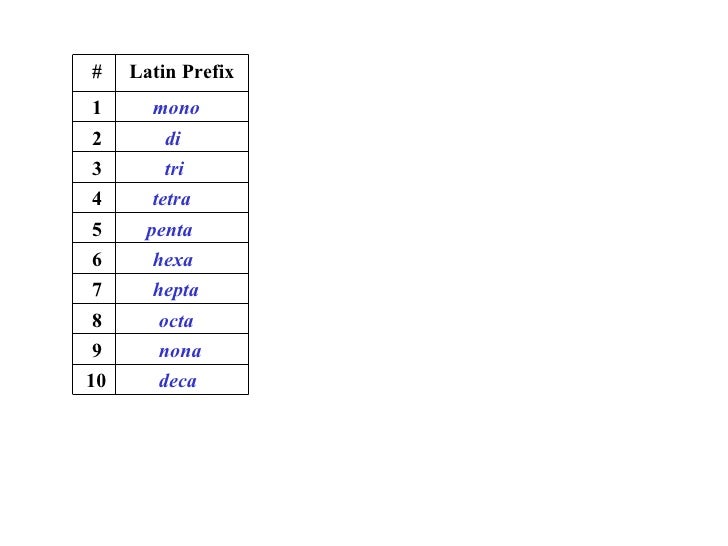
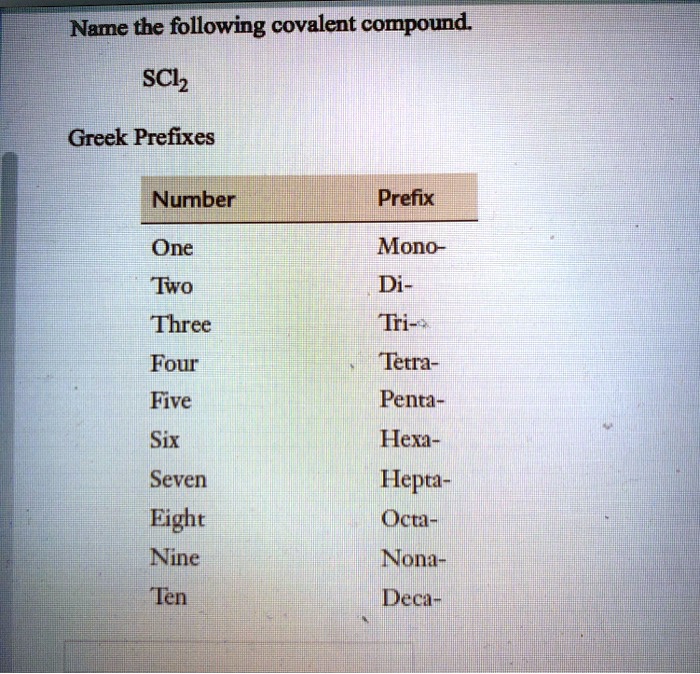
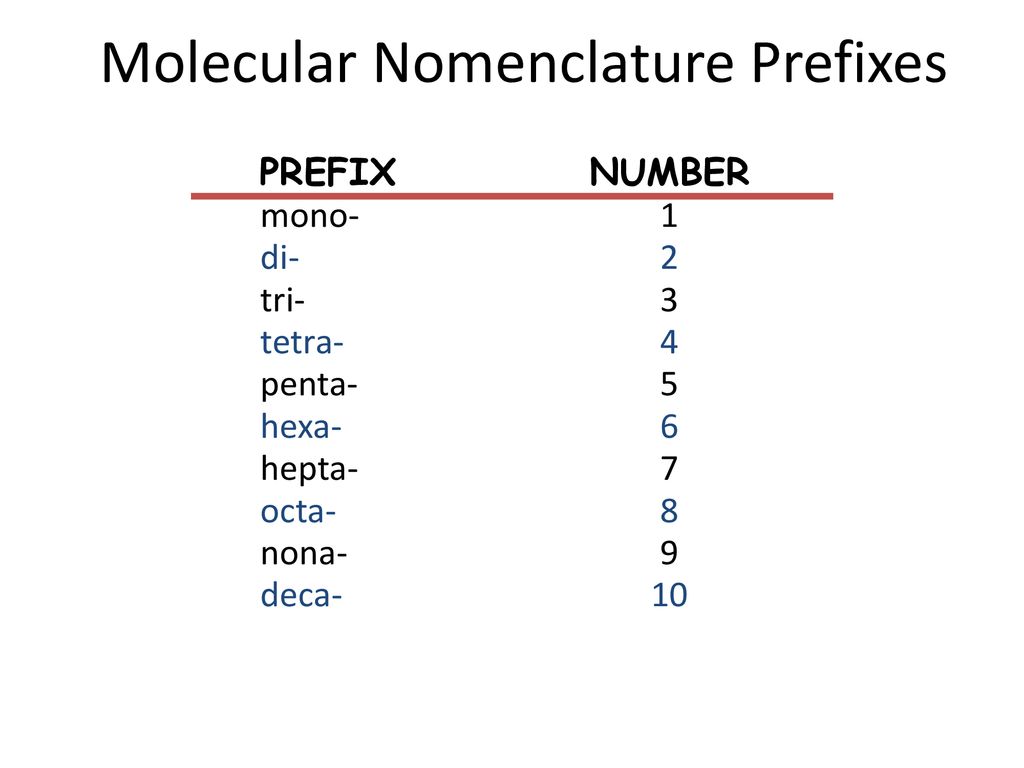
penta-5: hexa-6: hepta-7: octa-8: nona-9: deca-10: undeca-11: dodeca-12: If a molecule contains more than one atom of both elements, then prefixes are used for both. Thus N 2 O 3 is dinitrogen trioxide, as shown in Figure 6.1.1. In some names,. (omitting the a in tetra- according to step 2c).. 2 — di; 3 — tri; 4 — tetra; 5 — penta; 6 — hexa; 7 — hepta; 8 — octa; 9 — nona; 10 — deca; CCl 4 — carbon tetrachloride P 2 O 5 — diphosphorus pentoxide N 2 O — ninitrogen monoxide ICl 3 — iodine trichloride
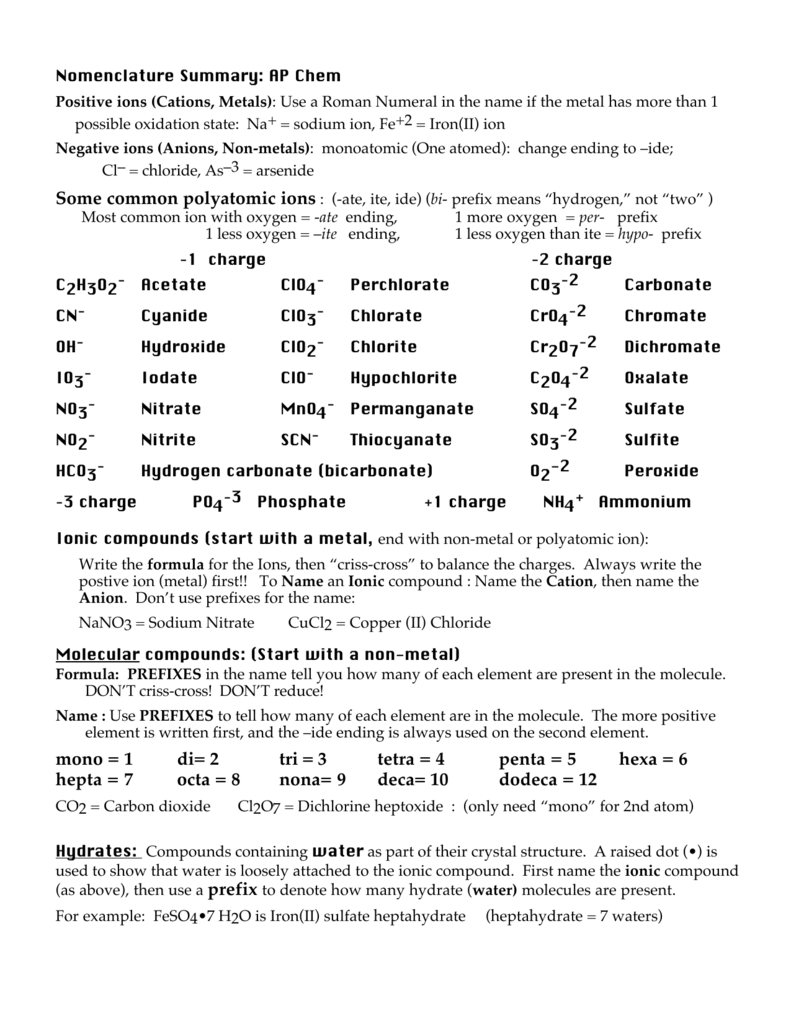
mono = 1 di= 2 tri = 3 tetra = 4 penta = 5 hexa = 6 hepta = 7 octa = 8
Blog Archives High School Biology and Chemistry
PPT II. Molecular Compounds (p. 178184, 227229) PowerPoint Presentation ID3953875
C20 Review Unit 01 Matter Energy And The Periodic Table

Binary Covalent & Acids ppt download
Mono Di Tri Tetra Penta Hexa De Onderdompeling In Cijfers

PPT In molecular bonding, molecules form, and they only include these atoms PowerPoint

Molecular Shapes VESPR Diagram Quizlet

WO2003037266A2 Tetra, penta, hexa and heptapeptides having antiangiogenic activity Google

Penta and hexacoordinated beryllium and phosphorus in highpressure modifications of CaBe2P2O8

Chem ch2 Diagram Quizlet

WO2003037266A2 Tetra, penta, hexa and heptapeptides having antiangiogenic activity Google

Tetra penta hecta shopslopez

PPT Formula writing PowerPoint Presentation, free download ID4011798

WO2003037266A2 Tetra, penta, hexa and heptapeptides having antiangiogenic activity Google
Naming covalent compounds and acids
Crystals Free FullText Tetra, Penta and HexaCoordinated Transition Metal Complexes
.PNG)
Naming Ionic Compounds
Covalent Nomenclature ppt download

Molecules & Compounds ? · mono di tri tetra penta hexa hepta octa nona deca III. Writing
di-3: tri-4: tetra-5: penta-6: hexa-7: hepta-8: octa-9: nona-10: deca-The rules for using the prefix system of nomenclature of binary compounds can be summarized as follows: Generally, the less electronegative element is written first in the formula, though there are a few exceptions. Carbon is always first in a formula and hydrogen is after.. Di-Tri-Tetra-Penta-Hexa-Hepta-Octa-Nona-Deca-Table \(\PageIndex{1}\): Common greek prefixes used in naming simple moleculs. There are some rules we need to follow,which are: If there are two atoms, place the more "metallic" first (furthest to the left on the periodic table)